Rising ETO Incidents in the Global Spice Trade: A Technical Guide for QC and HACCP Teams
Ethylene oxide (ETO) contamination in spices continues to disrupt global supply chains, with tightened regulations and recalls intensifying scrutiny. Following Hong Kong’s June 2025 alert on Cape Foods black pepper, this updated guide provides Quality Control (QC) and Hazard Analysis and Critical Control Points (HACCP) teams with detailed insights, technical data, and actionable strategies to ensure compliance and mitigate risks across major markets (US, EU, UK, Australia, Canada).
QUALITY CONTROLFOOD SAFETYREGULATIONSSTANDARDS
6/18/20255 min read


The Latest Incident: Cape Foods Black Pepper Alert (Hong Kong)
On June 17, 2025, Hong Kong’s Centre for Food Safety (CFS) issued a recall for a 170 g pack of “I Love Black Pepper with Grinders” (South African origin) after detecting ETO residues at 0.02 mg/kg, exceeding Hong Kong’s zero-tolerance limit (CFS, 2025). The affected batch was traced to inadequate post-fumigation aeration at a shared processing facility. Sales were halted, and a recall was initiated, underscoring the need for robust traceability systems.
Historical Context: Global ETO Alerts
ETO-related recalls have surged since 2023, driven by stricter regulations and improved detection technologies. Key incidents include:
April 2024: Singapore and Hong Kong banned Indian spice brands MDH and Everest after detecting ETO in spice blends at 0.3–0.5 mg/kg, exceeding Singapore’s 50 mg/kg limit for specific products but violating Hong Kong’s zero-tolerance policy (SFA, 2024; CFS, 2024).
2023–2024: Indian exporters adopted enhanced ETO testing (GC-MS and headspace analysis) to comply with EU/UK limits of 0.1 mg/kg, following rejections of 12% of spice consignments at EU borders (FSSAI, 2024).
Ongoing: Australia, Canada, Israel, Mexico, Russia, UAE, Vietnam, Indonesia, and Brazil have increased border inspections, with 15–20% of spice shipments tested for ETO residues in 2024–2025 (FAO, 2025).
Regional ETO Alerts in 2025
Recent incidents highlight the global scope of ETO contamination:
United States (FDA): March 2025 recall of 500 kg bulk turmeric powder in Massachusetts after FDA tests showed ETO at 0.15 mg/kg (limit 0.1 mg/kg; 21 CFR Part 117). The contamination stemmed from over-fumigation in a multi-product facility.
European Union (RASFF): April 2025 alert for Indian cumin seeds with ETO at 0.18 mg/kg (EU limit 0.1 mg/kg; Regulation (EC) No 396/2005). Shipments were redirected for retesting, with 30% failing secondary analysis.
United Kingdom (FSA): May 2025 notice on Turkish chilli flakes with ETO at 0.12 mg/kg (UK limit 0.1 mg/kg, aligned with EU). Retailers withdrew batches, citing inadequate supplier aeration logs.
Australia (FSANZ): February 2025 recall of coriander seed pods with ETO at 0.09 mg/kg, violating Australia’s zero-tolerance policy (FSANZ, 2025). The incident highlighted cross-contamination in packaging.
Canada (CFIA): May 2025 alert on Ontario-sold mixed spice packets with ETO at 0.14 mg/kg (limit 0.1 mg/kg; CFIA, 2025). A nationwide recall was enforced due to incomplete supplier records.
Understanding Ethylene Oxide (ETO): Technical Overview
Application: ETO (C₂H₄O, CAS 75-21-8) is a gaseous fumigant applied in sealed chambers (10–20 kPa, 40–50°C) to sterilize spices. Post-treatment aeration (25–30°C, 48–72 hours) reduces residues.
Purpose: Eliminates pathogens (e.g., Salmonella, E. coli) and molds without thermal degradation of volatile compounds (e.g., piperine in black pepper).
Contamination Risks:
Inadequate Aeration: Residual ETO binds to spice matrices, exceeding 0.1 mg/kg.
Over-Fumigation: Excessive ETO (e.g., >500 ppm) due to uncalibrated equipment.
Cross-Contamination: Shared fumigation chambers or packaging lines transfer ETO residues.
HACCP-Aligned Best Practices for ETO Control
Enhanced Testing Regimens:
Implement routine ETO residue testing using GC-MS (LOD 0.01 mg/kg) at raw material intake, post-fumigation, and pre-shipment stages.
Use third-party labs accredited to ISO/IEC 17025:2017 for export batches.
Apply statistical sampling (e.g., AOAC binomial sampling plans) to ensure 95% confidence in residue compliance.
Supplier Audits:
Audit fumigation facilities for equipment calibration, aeration protocols, and cleaning schedules (e.g., SOPs for chamber decontamination).
Verify supplier CoAs and ETO application logs against batch records.
Traceability Systems:
Deploy blockchain or QR-code tracking for lot-level traceability, enabling recall precision within 4 hours (Codex Alimentarius, 2020).
Integrate with ERP systems for real-time batch monitoring.
Alternative Sterilization Methods:
Steam Sterilization: Effective for whole spices (e.g., 120°C, 15 psi, 20 minutes). Validates pathogen reduction without chemical residues.
Irradiation: Gamma or electron beam (5–10 kGy) per Codex Standard 106-1983. Requires consumer labeling in some markets (e.g., EU, US).
Ozone Treatment: Emerging method (5–10 ppm ozone, 30 minutes) for surface sterilization. Limited by equipment cost and spice penetration.
Regulatory Monitoring:
Subscribe to real-time alerts from FDA, EU RASFF, UK FSA, FSANZ, and CFIA.
Use automated compliance software (e.g., FoodLogiQ, TraceGains) to track regulatory updates across 20+ markets.
Turning Compliance into Competitive Advantage
Proactive ETO management strengthens supply chain resilience and brand reputation. By integrating advanced testing (GC-MS, LC-MS/MS), robust HACCP plans, and alternative sterilization, spice processors can:
Reduce recall risks by 80% through pre-shipment testing (FAO, 2025).
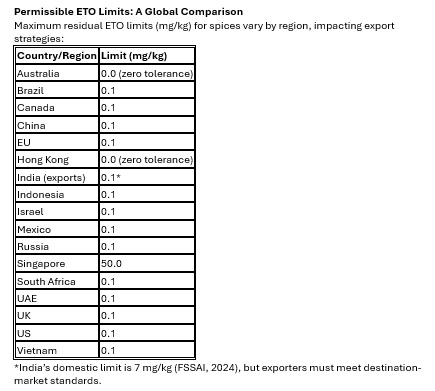
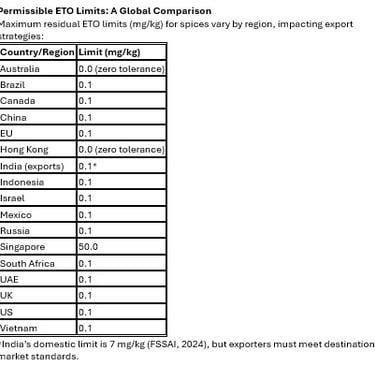
Meet multi-market compliance (0.0–0.1 mg/kg limits) with a single SOP.
Build consumer trust via transparent CoAs and traceability systems.
Advanced Tools and Future Trends
Residue Testing Technologies:
Headspace GC-MS: Detects volatile ETO residues with LOD of 0.005 mg/kg, ideal for low-residue markets (Australia, Hong Kong).
Portable FTIR Spectroscopy: Emerging for on-site ETO screening (accuracy ±0.02 mg/kg), reducing lab dependency.
Predictive Analytics: Machine learning models (e.g., trained on RASFF data) predict high-risk suppliers with 85% accuracy (EFSA, 2025).
Regulatory Outlook: The EU and US are exploring lower ETO limits (0.05 mg/kg) by 2027, while Australia may mandate alternative sterilization for imports.
For further guidance, contact regulatory bodies or explore advanced testing solutions at industry events like IFT 2026.
Technical Notes for QC/HACCP:
Traceability Requirement: Implement batch-level tracking (e.g., QR codes or blockchain) to isolate affected lots within 24 hours, as required by ISO 22000:2018.
Regulatory Trend: Global harmonization toward a 0.1 mg/kg limit (except Australia and Hong Kong’s zero tolerance) requires pre-export testing. Use accredited labs compliant with ISO/IEC 17025:2017.
Risk Assessment: Conduct a hazard analysis for ETO as a chemical contaminant under HACCP principles (Codex Alimentarius, 2020). Identify raw material sourcing and fumigation as high-risk stages.
Testing Protocols: Use GC-MS or liquid chromatography-tandem mass spectrometry (LC-MS/MS) with LOD ≤ 0.01 mg/kg. Validate methods against AOAC International standards (e.g., AOAC 2019.01).
CCP Monitoring: Establish CCPs at raw material intake, fumigation, and packaging. Monitor ETO residues at each stage using in-house or third-party labs.
Corrective Actions: For non-compliant batches, implement re-aeration (72 hours, 25°C, 500 m³/h airflow) or diversion to markets with higher limits (e.g., Singapore).
Compliance Strategy: Align production with the strictest market (Australia/Hong Kong: 0.0 mg/kg) to simplify export processes. Use statistical process control (SPC) to monitor residue variability.
Documentation: Maintain certificates of analysis (CoA) for each batch, specifying ETO testing method, LOD, and compliance with destination regulations.
Risk Mitigation: Segregate ETO-treated and non-treated spices in storage and processing to prevent cross-contamination.
Training: Train QC staff on ETO testing methods and HACCP principles annually, per ISO 22000:2018.
KPI Metrics: Track ETO compliance rate (target: 99.9%), recall incidents (target: 0/year), and supplier audit pass rate (target: 95%).
Continuous Improvement: Use Plan-Do-Check-Act (PDCA) cycles to refine ETO controls, integrating feedback from regulatory rejections.
Equipment Investment: Budget for GC-MS systems ($50,000–$100,000) or partner with accredited labs to meet testing demands.
Data Integration: Use IoT sensors in fumigation chambers to feed real-time ETO data into HACCP dashboards.
References:
CFS (2025). Food Safety Alert: Black Pepper Recall.
Codex Alimentarius (2020). HACCP Guidelines.
EFSA (2025). Predictive Analytics for Food Safety.
FAO (2025). Global Spice Trade Report.
FSSAI (2024). Spice Export Regulations.
ISO 11135:2014. Sterilization of Health Care Products.
ISO 22000:2018. Food Safety Management Systems.
Notice any errors? Please help us rectify to get free sample!
White Label / Private Label Services
Welcome White Label / Private Label inquiries with full customization, control over Quality/Safety, and ensuring excellence in processing, packaging, and exporting of products.
Tel: +852 25172316
© 2024. All rights reserved. All information on this site is without any contractual, financial or otherwise any other liability. For binding information, please contact us and include your concerns / requirements in enquiry, quotation, contract and shipping documents.
5/Fl. Qualipak Tower, 122 Connaught Road West
Hong Kong.
China Business Ltd. T/A Orient Resources Company


Fax: +852 25178741
